used in titration of the impregnite sample. Calculate the
(3) Procedure. Determine the active chlorine in
percentage active chlorine as follows:
the impregnite as follows:
(a) Chloroform used as a solvent will
U.S.P. or C.P. grade and purified on the same day it is
to be used as follows: Place 1 liter the chloroform in a 2-
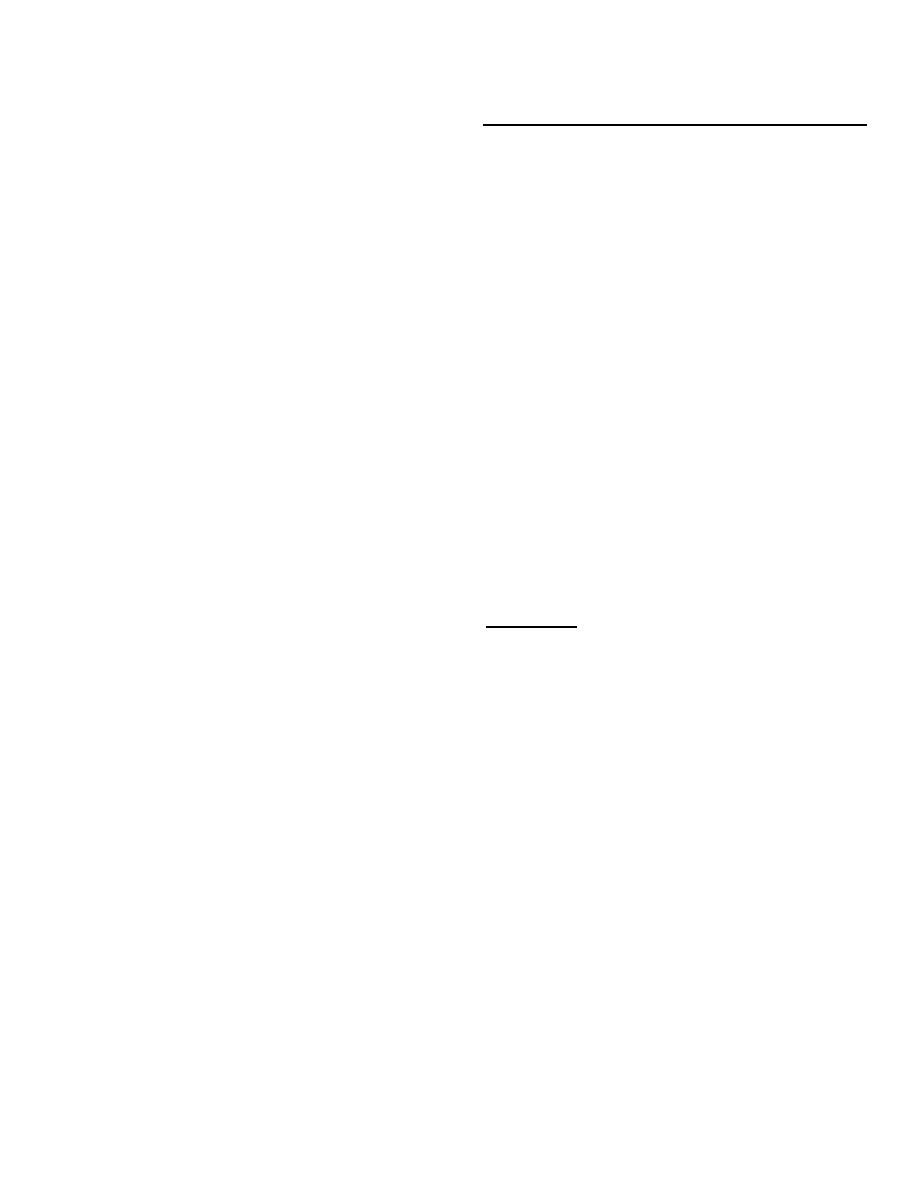
Weight of sample
liter separatory funnel 2 shake with four changes of
= percent active chlorine
distilled water, 500 each. Filter the washed chloroform
through two thicknesses of dry filter paper. To 50 ml of
b. Chlorinated Paraffin Test.
chloroform add a freshly prepared potassium iodide
(1) Requirement.
HC1 (hydrochloric acid)
solution, consisting of 45 ml of distilled water, 2 grams
content in the chlorinated paraffin shall not be greater
of potassium iodide, and 5 ml 1:1 hydrochloric acid.
than 0.10 percent when tested.
(Dissolve the potassium iodide in the distilled water and
(2) Equipment required. Standard chemical
then add hydrochloric acid.) Add starch indicator X
laboratory equipment as described in "Procedure".
shake vigorously for 5 minutes. After shaking blue or
(3) Procedure.
faint blue color should appear. Failure of the color to
(a) Weigh 20 grams of the sample and
appear reveals presence of reducing agent which
transfer to a 250 ml separatory funnel. Add 50 ml of
indicates the chloroform unsuitable as a solvent and
benzine and shake thoroughly. Add 30 ml of 95 percent
must not be used. If the desired blue color is obtained
ethyl alcohol, shake, add 100 ml of water and shake
titrate with 0.1N sodium thiosulfate solution. If a blank
thoroughly. Allow mixture to stand until layers separate.
greater than 0.20 ml of 0.1N sodium thiosulfate is
(b) Separate alcohol-water solution and add
obtained, repurify the chloroform as above adding 10
a few drops of mixed indicator solution. The indicator
grams of potassium iodide to the first wash water.
consists of 0.030 grams of methyl red and 0.075 grams
of
bromcresol
green
(tetrabromo-m-cresol-
(b) Break up all lumps present in
sulfonphthalein) dissolved on 100 ml of 95 percent ethyl
impregnite and pass the entire sample through No. 30
alcohol.
U.S. standard sieve. Return the sample to the bottle.
(c) Titrate with 0.02 normal NaOH (sodium
Weigh from 0.55 to 0.60 grams the uniform sample and
hydroxide) to the intermediate purple end point.
wash it through a fun into a 300-ml glass stoppered
(d) Run a blank using the same procedure, with
iodine flask w 50 ml of purified chloroform. Stopper the
the same volumes of benzine, alcohol, and water, but
flask and agitate gently to dissolve the sample. As soon
no chlorinated paraffin and titrate as above.
as the sample has dissolved, add a freshly p pared
(e) Calculate the HC1 (hydrochloric acid)
potassium iodide solution consisting of ml of distilled
content as follows: % HC1 (hydrochloric acid) =
water, 2 grams of potassium iodide and 5 ml of 1:1
M x N x 3.65
hydrochloric acid. (Dissolve potassium iodide, in the
W
distilled water and t} add the hydrochloric acid.) Stopper
the flask E shake vigorously for 5 minutes. Cool the
Where: M =
Number of ml NaOH less correction for
flask cold water, open, and wash the stopper and walls
blank
of the flask with distilled water. Titrate the Iiberated
N =
iodide with 0.1N sodium thiosulfate solution. During the
W =
Weight of chlorinated paraffin in grams
titration, swirl the contents the flask continuously and
vigorously, so there will always be an excess of iodine in
the acid-acqueous layer, until the final end point is reach
7. Documentation. a. Report Forms. When reporting
Near the end point, stopper and shake the flask
data, the following forms will be used:
vigorously after each addition of thiosulfate. When the
iodine color has almost disappeared, a starch indicator
DA Form 984 .....Munitions Surveillance Report.
DA Form 985 .....Data Sheet for Grand Lots, Miscellaneous
and titrate to the disappears of the blue color.
Lots or Depot Lots.
Determine a blank on 50 ml the chloroform by the same
DA Form 2028 ...Recommended
Changes
to
DA
procedure gin above. Subtract the ml of sodium
Publications.
thiosulfate solution used in titration of the blank from ml
b. Reporting.
(1) Data. When reporting data, forms specified
in 7a will be prepared in accordance with instructions
contained in SB 3-30, SB 742-1 and TM 38-750.
4


