01 March 2005
NAVAIR 01-1A-509-1
TM 1-1500-344-23-1
TO 1-1-689-1
Corrosion Products
Electrolyte
Steel
Fastener
(Cathode)
Magnesium Alloy
(Anode)
Figure 3-8. Galvanic Corrosion of Magnesium
Adjacent to Steel Fastener
Figure 3-7. Surface Corrosion on Frequency Test Set
3-9. T Y P E S O F C O R R O S I O N . C o r r o s i o n i s
catalogued and typed in many ways. Occasionally,
different names are used for the same type of corrosion.
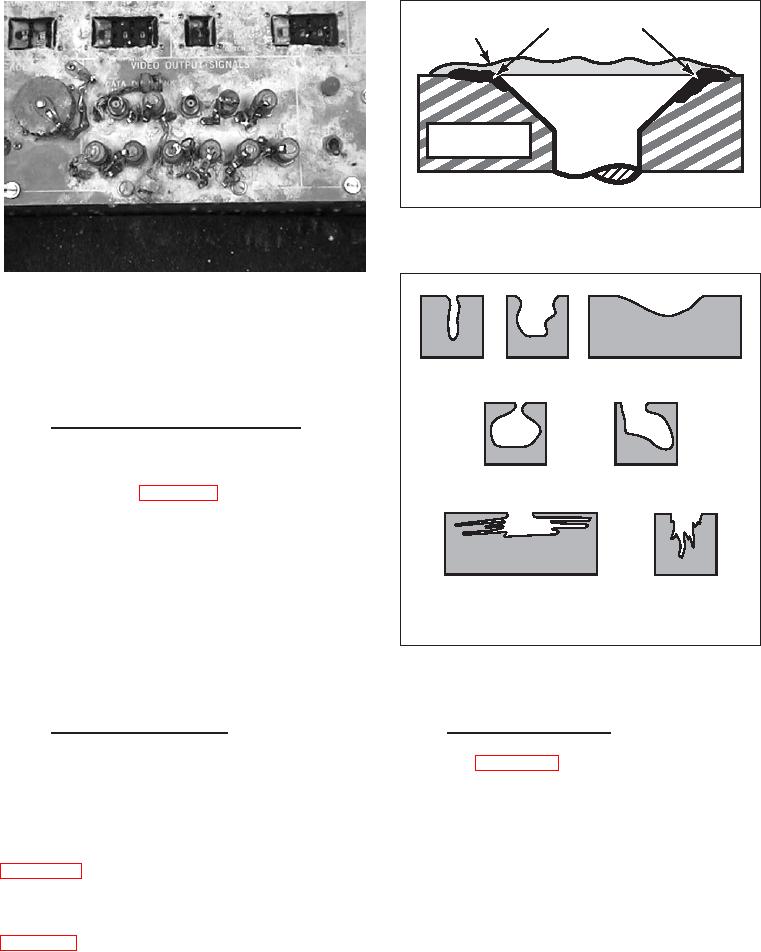
Narrow &
Elliptical
Wide and Shallow
Deep
The common types of corrosion are described below.
3-9.1. UNIFORM SURFACE CORROSION. Uniform
surface corrosion is probably the most common type of
corrosion. It results from a direct chemical attack on a
Subsurface
Undercutting
metal surface that proceeds uniformly over the entire
exposed surface (see Figure 3-7). The metal gradually
becomes thinner and eventually fails. On a polished
surface, this type of corrosion is first seen as a general
dulling or etching of the surface and, if the attack is
allowed to continue, the surface becomes rough and
possibly frosted in appearance. An example is the
Horizontal*
Vertical*
etching of metals by acids. The discoloration or general
dulling of metal created by exposure to elevated
* Shapes determined by microstructural orientation
temperatures is not considered to be uniform surface
corrosion. Coating/sealing the exposed surface will
Figure 3-9. Variations in the Cross-Sectional
protect it from this type of attack. Also, corrosive elements
Shape of Corrosion Pits
may be removed through air movement and drain holes.
3-9.2. GALVANIC CORROSION. Galvanic corrosion
3-9.3. PITTING CORROSION. Pitting is a form of
occurs when different metals are in contact with each
extremely localized attack that results in holes in the
other and an electrolyte, such as sea water. It is usually
metal (see Figure 3-9). Pits can be isolated, or so
recognizable by the presence of a buildup of corrosion
close together that they look like a rough surface. Pits
deposits at the joint between the metals. For example,
are often difficult to detect because of their small size
aluminum skin panels and stainless steel doublers,
and because they may be covered with corrosion
riveted together in an aircraft wing, form a galvanic
products. Pitting is usually first noticeable as a white or
couple if moisture and contamination are present.
gray powdery deposit, similar to dust, which blotches
Figure 3-8 shows galvanic corrosion of magnesium
the surface. When the deposit is cleaned away, tiny
adjacent to steel fasteners. The potential for galvanic
pits or holes can be seen in the surface. Most pits
corrosion is greatest when the two metals are well
develop and grow downward (in the direction of gravity)
separated from each other in the galvanic series (see
from a horizontal surface. Pitting failures are commonly
Figure 3-4) and are in electrical contact.
caused by electrolytes containing chloride or chlorine-

