01 March 2005
NAVAIR 01-1A-509-1
TM 1-1500-344-23-1
TO 1-1-689-1
Electrolyte Enters
Through Cracks in
Intergranualar
Paint Film
Paint Film
Corrosion
Steel
Fastener
(Cathode)
7075-T6 Aluminum
(Anode)
Figure 3-14. Extreme Example of Exfoliation at
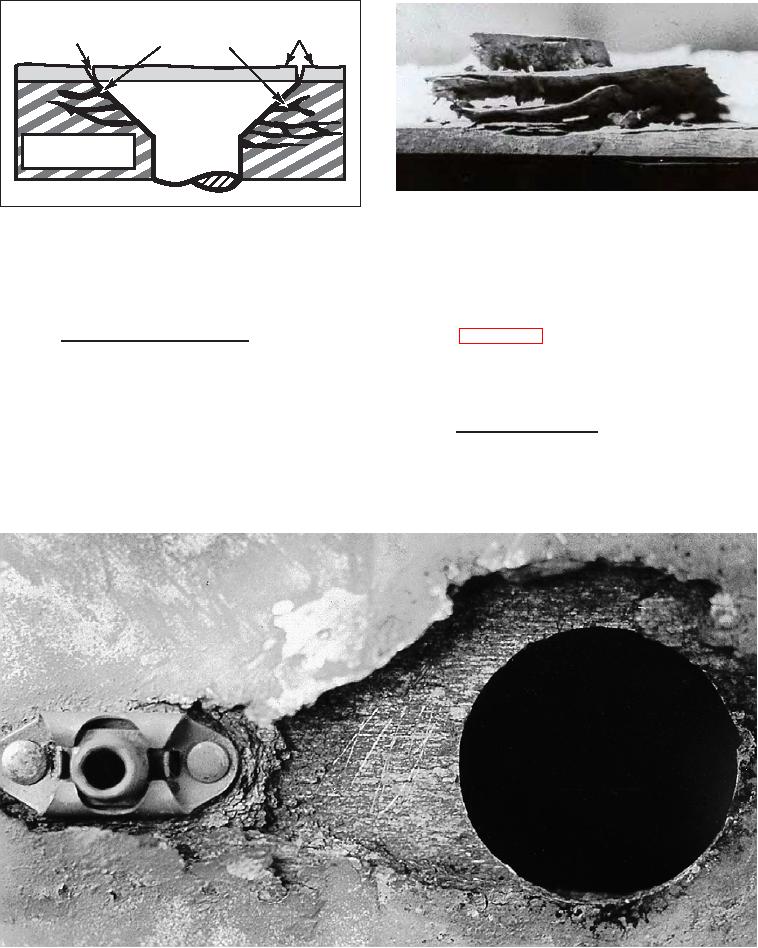
Figure 3-13. Intergranular Corrosion of 7075-T6
Edge of Sheet
Aluminum Adjacent to Steel Fasterner
by welding, sealant, or soldering, and use of
wood, rubber, plastic tape, and other materials in
nonabsorbent gaskets (such as Teflon).
contact with the metal surface. Corrosion will occur at
the area of low oxygen concentration (anode) as
3-9.6.1. Oxygen Differential Cells. Electrolyte in
illustrated Figure 3-16, View A. Alloys such as stainless
contact with metal surfaces will normally contain
steel, which owe their corrosion resistance to surface
dissolved oxygen. An oxygen cell can develop at any
passivity, are particularly susceptible to this type of
point where the oxygen in the air is not allowed to
crevice corrosion.
diffuse into the solution, thereby creating a difference
in oxygen concentration between two points. Typical
3-9.6.2. Active/Passive Cells. Metals which depend
locations of oxygen differential cells are under either
on a tightly adhering passive film, such as the oxide
metallic or non-metallic deposits (dirt) on the metal
film on corrosion resistant steel, are prone to rapid
surface and under faying surfaces such as riveted lap
corrosive attack by active/passive cells. The corrosive
joints. Oxygen cells can also develop under gaskets,
action usually starts with a deposit of dirt or salt which
Figure 3-15. Exfoliation Adjacent to Fasteners

