01 March 2005
NAVAIR 01-1A-509-1
TM 1-1500-344-23-1
TO 1-1-689-1
No Contact
Between
Unbroken
Electrolyte &
Paint Film
Anode &
Electrolyte
Cathode
(Fresh or Sea Water,
Acids, Gases)
Electrolyte
(Continuous Liquid Path)
Anodic
Cathodic
Area
Area
Cathodic
Anodic
Area
Area
Ele
ctron Flow
Metal
Metal
Figure 3-2. Elimination of Corrosion by Application
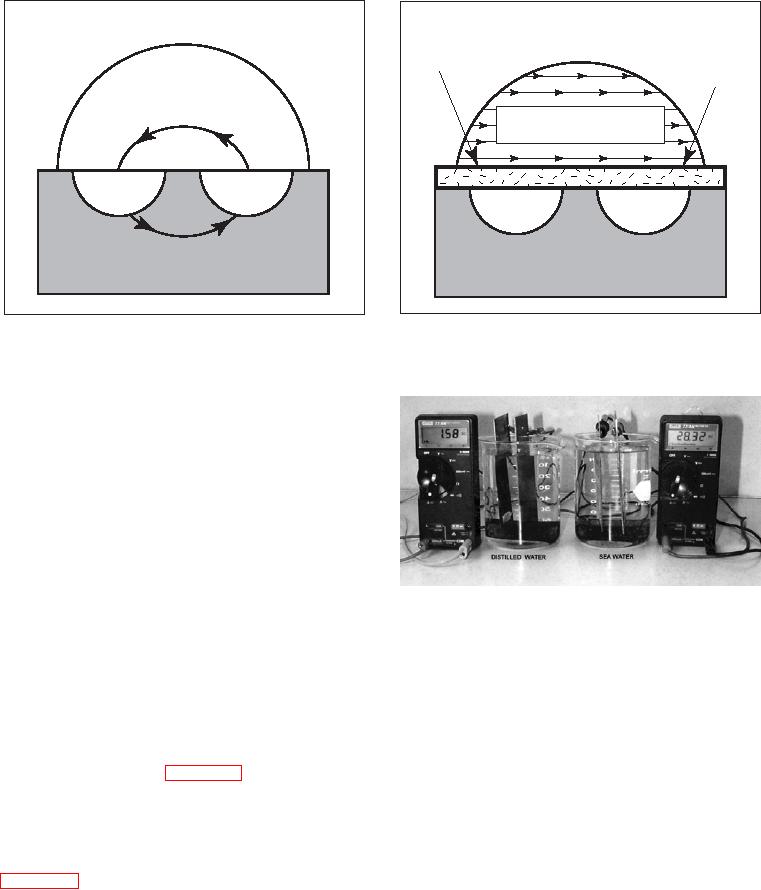
Figure 3-1. Simplified Corrosion Cell
of an Organic Film to a Metal Surface
a. A metal which has a tendency to corrode must
be present (the corroding metal is called the anode);
b. A dissimilar conductive material (the cathode),
which has less tendency to corrode than the anode,
must be present (a dissimilar metal may be a different
metal, a protected part of the same metal, or conductive
plastic);
c. A conductive liquid (electrolyte) must connect the
anode and cathode (so that ions can carry electric
current between them); and
Figure 3-3. Effect of Sea Water on
d. Electrical contact between the anode and cathode
Galvanic Corrosion
(usually in the form of metal-to-metal contact) must
exist so that electrons can move between the anode
corrosion begins on an inside surface of a component
and the cathode.
(for example, the inner wall of a metal tube), it may go
undetected until perforation occurs.
3-6.2. The elimination of any one of the four conditions
will slow or stop corrosion. For example, a paint film on
3-7.1. When corrosion products form, they often
a metal surface will prevent the electrolyte from
precipitate onto the corroding surface as a powdery
connecting the anode and cathode, thereby stopping
deposit. This film of corrosion products may reduce the
the electric current (see Figure 3-2). A change in the
rate of corrosion, if the film acts like a barrier to
electrolyte can also affect the rate of corrosion. Two
electrolytes. Some metals (such as stainless steel and
connected dissimilar metal parts placed in distilled
titanium), under the right conditions, produce corrosion
water corrode very slowly due to a lack of ions in solution
products that are so tightly bound to the corroding metal
to conduct the electric current; in sea water the corrosion
that they form an invisible oxide film (called a passive
reaction is accelerated by a factor of 1000 or more (see
film) which prevents further corrosion. However, when
the film of corrosion products is loose and porous (such
as those of aluminum and magnesium), an electrolyte
3-7. DEVELOPMENT OF CORROSION. All corrosive
can easily penetrate and continue the corrosion process,
attack begins on the surface of the metal. If allowed to
producing more extensive damage than surface
progress, corrosion can penetrate into the metal. If
appearance would indicate.

